Key points
- One inactivated Japanese encephalitis (JE) vaccine (manufactured as IXIARO) is available in the United States.
- JE vaccine is approved for use in children aged 2 months and older and adults.
- JE is a very low risk disease for most travelers.
- JE vaccine is recommended or should be considered for some travelers based on their duration of travel, time and location of travel, and planned activities.
Japanese encephalitis vaccine
One inactivated Vero cell culture-derived Japanese encephalitis (JE) vaccine (called IXIARO and manufactured by Valneva) is available in the United States. This vaccine was approved in March 2009 for use in people aged 17 years and older and in May 2013 for use in children 2 months through 16 years of age. Other JE vaccines are manufactured and used in other countries but are not licensed for use in the United States.
For adults and children aged 3 years or older, each dose of IXIARO is 0.5 mL. For children aged 2 months through 2 years, each dose is 0.25 mL.
IXIARO is given as a two-dose series, with the doses spaced 28 days apart. Adults aged 18–65 years can get the second dose from 7–28 days after the first dose. The last dose should be given at least 1 week before travel.
A booster dose (third dose) should be given if a person has received the two-dose primary vaccination series one year or more previously and there is a continued risk for JE virus infection or potential for reexposure.
More information about JE vaccine can be found in the Vaccine Information Statement for IXIARO Japanese Encephalitis Vaccine [PDF – 2 pages] and in the IXIARO product information available at the FDA IXIARO web page.
Risk factors for JE among travelers
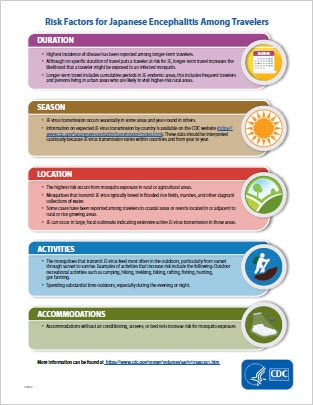
JE is a very low risk disease for most travelers to JE-endemic countries. However, some travelers will be at increased risk of infection based on factors including longer periods of travel, travel during the JE virus transmission season, spending time in rural areas, participating in a lot of outdoor activities, and staying in accommodations without air conditioning, screens, or bed nets.
Risk factors for Japanese encephalitis among travelers
Considerations for JE vaccination for travelers
Healthcare providers should advise all travelers to JE-endemic countries to take steps to avoid mosquito bites and discuss the need for vaccination. The discussion should include the risks related to the specific travel itinerary, the likelihood of future travel to countries where JE occurs, the possible severe outcomes of JE disease, and information about the vaccine including cost and possible side effects.
JE vaccine recommendations
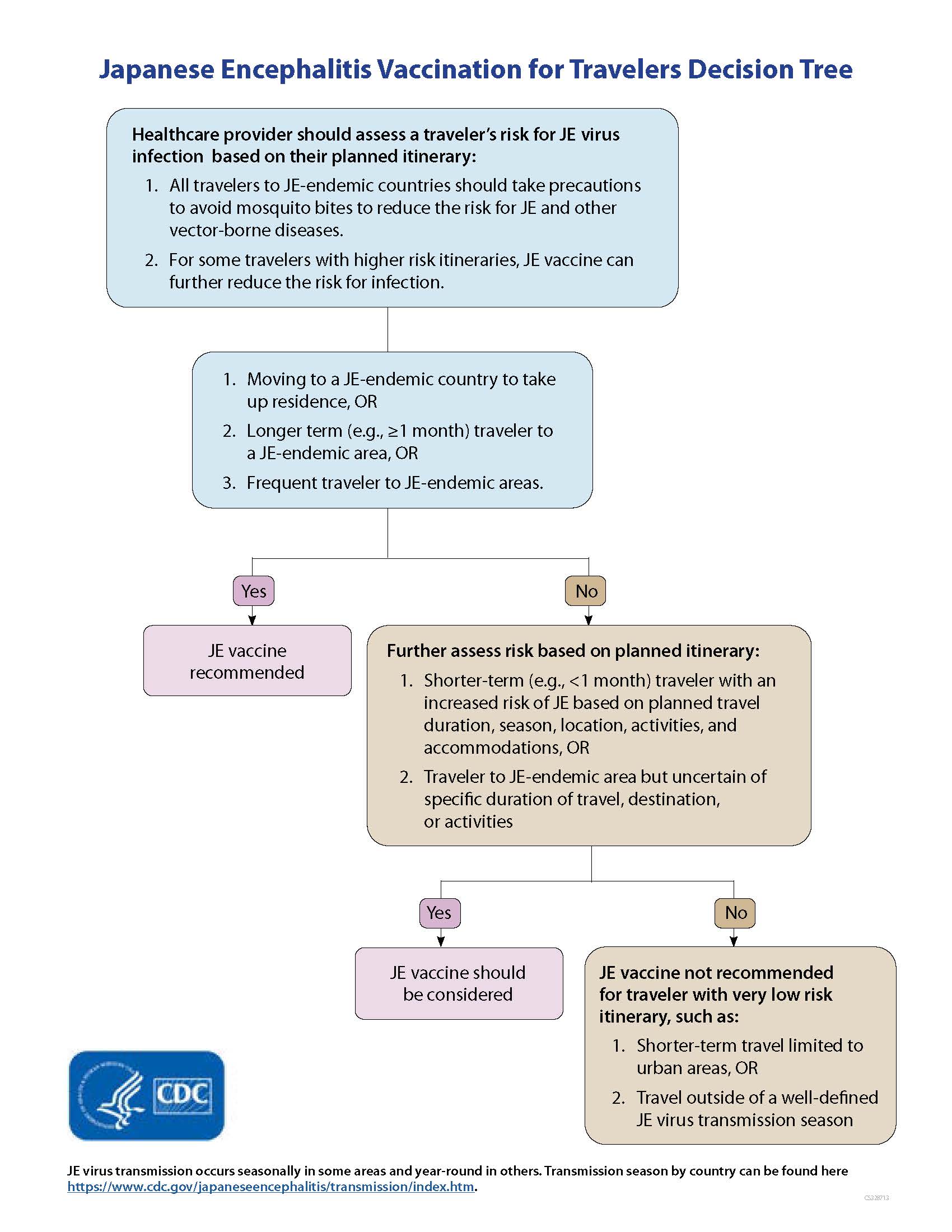
- JE vaccine is recommended for persons moving to a JE-endemic country to live, longer-term (e.g., 1 month or longer) travelers, and frequent travelers to JE-endemic areas.
- JE vaccine also should be considered for shorter-term (e.g., less than 1 month) travelers with an increased risk of JE based on planned travel duration, season, location, activities, and accommodations, and for travelers to endemic areas who are uncertain of specific duration of travel, destinations, or activities.
- JE vaccine is not recommended for travelers with very low risk itineraries, such as shorter-term travel limited to urban areas or travel that occurs outside of a well-defined JE virus transmission season.
Healthcare providers may use CDC's decisions tree to help determine when Japanese encephalitis vaccination for travelers is appropriate.
Japanese encephalitis vaccination for travelers decision tree
Precautions and contraindications
- A severe allergic reaction (e.g., anaphylaxis) after a previous dose of IXIARO, any other JE vaccine, or any component of IXIARO is a contraindication to further doses. The vaccine contains protamine sulfate, a compound known to cause allergic reactions in some people.
- Administration of IXIARO to pregnant women usually should be deferred. However, pregnant women who must travel to an area where risk for infection is high should be vaccinated when the theoretical risk of immunization is outweighed by the risk of infection.
Side effects of JE vaccine
Reactions to IXIARO are generally mild and include pain and tenderness at the injection site, headache, myalgia, and low-grade fever.
Healthcare providers are encouraged to report all adverse events that might be caused by vaccination to the CDC/FDA Vaccine Adverse Events Reporting System (VAERS) by one of the following methods.
- Submit a report online.
- Print a VAERS form and upload as instructed.
If you need further assistance with reporting to VAERS, please email info@VAERS.org or call 1-800-822-7967.
For more information
- MMWR Recommendations and Reports: Advisory Committee on Immunization Practices (ACIP) recommendations for use of JE vaccine
- CDC's Health Information for International Travel (Yellow Book): Information about JE risk by country and recommendations for prevention of JE among travelers
- Rabe IB, Miller ER, Fischer M, Hills SL. Adverse events following vaccination with an inactivated, Vero cell culture-derived Japanese encephalitis vaccine in the United States, 2009-2012. Vaccine. 2015 Jan 29;33(5):708-12. doi: 10.1016/j.vaccine.2014.11.046
- Schuller E, Klingler A, Dubischar-Kastner K, Dewasthaly S, Müller Z. Safety profile of the Vero cell-derived Japanese encephalitis virus (JEV) vaccine IXIARO (®). Vaccine. 2011;29(47):8669-76. doi: 10.1016/j.vaccine.2011.08.117
- Tauber E, Kollaritsch H, von Sonnenburg F, Lademann M, Jilma B, Firbas C, et al. Randomized, double-blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis. 2008;198(4):493–9. doi: 10.1086/590116
- Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet. 2007;370(9602):1847–53. doi: 10.1016/S0140-6736(07)61780-2
- Woolpert T, Staples JE, Faix DJ, Nett RJ, Kosoy O, Biggerstaff BJ, et al. Immunogenicity of one dose of Vero cell culture-derived Japanese encephalitis (JE) vaccine in adults previously vaccinated with mouse brain-derived JE vaccine. Vaccine. 2012;30(20):3090-3096. doi: 10.1016/j.vaccine.2012.02.063
