Demographic and Clinical Characteristics of Mpox in Persons Who Had Previously Received 1 Dose of JYNNEOS Vaccine and in Unvaccinated Persons — 29 U.S. Jurisdictions, May 22–September 3, 2022
Weekly / December 30, 2022 / 71(5152);1610–1615
Please note:. This report has been corrected.
Jennifer L. Farrar, MPH1; Nathaniel M. Lewis, PhD1; Kennedy Houck, MPH1; Michelle Canning, MPH1; Amy Fothergill, PhD1,2; Amanda B. Payne, PhD1; Adam L. Cohen, MD1; Joshua Vance, MPH, MEd3,4; Bridget Brassil, MPH5; Erin Youngkin, MPH6; Bailey Glenn, MS7,8; Anil Mangla, PhD9; Nikki Kupferman, MS10; Katharine Saunders, DNP2,11; Cristina Meza, MPH12; Dawn Nims, MPH13; Susan Soliva, MPH14; Brandon Blouse, MPH15; Tiffany Henderson, MPH16; Emily Banerjee, MPH17; Brooklyn White, MPH18; Rachael Birn, MPH8,19; Anna M. Stadelman, PhD2,20; Meaghan Abrego, MPH21; Meagan McLafferty, MPH22; Michael G. Eberhart, MPH23; Michael Pietrowski, MPH24; Sandra Miranda De León, MPH25; Emma Creegan, MPH26; Abdoulaye Diedhiou, MD, PhD27; Caleb Wiedeman, MPH28; Jade Murray-Thompson, MPH29; Elizabeth McCarty, MPH30; Jessica Marcinkevage, PhD31; Anna Kocharian, MS32; Elizabeth A. Torrone, PhD1; Logan C. Ray, MPH1; Daniel C. Payne, PhD1; Mpox Cases in Vaccinated Persons Team (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Evidence suggests that 1 dose of JYNNEOS vaccine offers some protection against monkeypox (mpox).
What is added by this report?
Analysis of mpox infections among unvaccinated persons and those who had received 1 JYNNEOS vaccine dose ≥14 days before illness onset found that the odds of fever, headache, malaise, myalgia, and chills were significantly lower among vaccinated patients than among unvaccinated patients. Overall, 2% of vaccinated persons with mpox and 8% of unvaccinated patients were hospitalized.
What are the implications for public health practice?
One dose of JYNNEOS vaccine might attenuate the severity of illness and reduce hospitalization in persons who become infected after vaccination; however, to optimize protection, all eligible persons are recommended to complete the 2-dose vaccination series.
As of November 14, 2022, monkeypox (mpox) cases had been reported from more than 110 countries, including 29,133 cases in the United States.* Among U.S. cases to date, 95% have occurred among males (1). After the first confirmed U.S. mpox case on May 17, 2022, limited supplies of JYNNEOS vaccine (Modified Vaccinia Ankara vaccine, Bavarian Nordic) were made available to jurisdictions for persons exposed to mpox. JYNNEOS vaccine was approved by the Food and Drug Administration (FDA) in 2019 as a 2-dose series (0.5 mL per dose, administered subcutaneously) to prevent smallpox and mpox disease.† On August 9, 2022, FDA issued an emergency use authorization to allow administration of JYNNEOS vaccine by intradermal injection (0.1 mL per dose) (2). A previous report on U.S. mpox cases during July 31–September 3, 2022, suggested that 1 dose of vaccine offers some protection against mpox (3). This report describes demographic and clinical characteristics of cases occurring ≥14 days after receipt of 1 dose of JYNNEOS vaccine and compares them with characteristics of cases among unvaccinated persons with mpox and with the vaccine-eligible vaccinated population in participating jurisdictions. During May 22–September 3, 2022, among 14,504 mpox cases reported from 29 participating U.S. jurisdictions,§ 6,605 (45.5%) had available vaccination information and were included in the analysis. Among included cases, 276 (4.2%) were among persons who had received 1 dose of vaccine ≥14 days before illness onset. Mpox cases that occurred in these vaccinated persons were associated with lower percentage of hospitalization (2.1% versus 7.5%), fever, headache, malaise, myalgia, and chills, compared with cases in unvaccinated persons. Although 1 dose of JYNNEOS vaccine offers some protection from disease, mpox infection can occur after receipt of 1 dose, and the duration of protection conferred by 1 dose is unknown. Providers and public health officials should therefore encourage persons at risk for acquiring mpox to complete the 2-dose vaccination series and provide guidance and education regarding nonvaccine-related prevention strategies (4).
Probable and confirmed mpox cases¶ among persons with illness onset during May 22–September 3, 2022, in the 29 jurisdictions were eligible for inclusion. Persons who had received 1 dose of JYNNEOS vaccine ≥14 days before illness onset were considered vaccinated for the purposes of this study**; those who had not received 1 vaccine dose during the current outbreak or who reported illness onset before receipt of their first vaccine dose were considered unvaccinated. Cases were excluded if 1) no vaccination date or vaccination status was available, 2) receipt of vaccine occurred before May 2022, or 3) illness onset occurred ≤13 days after receipt of 1 vaccine dose.
Participating jurisdictions collected data using a standardized data collection form†† including self-reported demographic characteristics, vaccination history, medical history, and possible exposures. Participating jurisdictions linked vaccination data from immunization registries when available or by self-report during case investigation and transmitted the linked data to CDC.
Demographic characteristics of persons with mpox who had received 1 vaccine dose (i.e., were vaccinated) were compared with those of unvaccinated persons with mpox. In addition, characteristics of persons with mpox who were vaccinated were compared with those of all vaccine-eligible persons who were vaccinated, irrespective of case status; these data were obtained through jurisdictional immunization registries reporting first doses administered. Comparisons were made using Pearson’s chi-square test, Fisher’s exact test, or the Wilcoxon rank-sum test as appropriate. P-values <0.05 were considered statistically significant.
To assess differences in illness among vaccinated and unvaccinated persons with mpox, clinical characteristics were compared between these two groups among persons with data reported for one or more clinical symptoms. Missing individual symptom data were imputed as “no” when there was evidence that the reporting jurisdiction collected symptom data in a “check-all-that-apply” format.§§ Odds ratios and 95% CIs were calculated to compare clinical characteristics of vaccinated and unvaccinated mpox patients. Persons with missing data for relevant variables of interest were excluded from individual analyses.
Because JYNNEOS vaccine was administered for postexposure prophylaxis at the beginning of the outbreak, and because the mpox incubation period can be as long as 21 days, a sensitivity analysis including only persons with mpox who received 1 vaccine dose ≥22 days before illness onset as the vaccinated group was conducted. SAS (version 9.4; SAS Institute) and R (version 4.0.3; R Foundation) were used to conduct all analyses. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.¶¶
During May 22, 2022–September 3, 2022, a total of 14,504 mpox cases were reported in the 29 included jurisdictions. Among the 6,605 (45.5%) persons with mpox who had available information and who were included in the analysis,*** 6,329 (95.8%) were unvaccinated, and 276 (4.2%) had illness onset ≥14 days after receiving 1 vaccine dose (Table 1). Among vaccinated patients, the median interval from vaccination to illness onset was 23 days (IQR = 17.5–30 days). The age distribution differed for vaccinated mpox patients compared with the vaccine-eligible population identified through immunization registries (p<0.001), but not compared with unvaccinated mpox patients (p = 0.07); 91.2% of vaccinated mpox patients were aged 18–49 years. Overall, 68.2% of vaccinated mpox patients and 49.9% of unvaccinated mpox patients reported White race, whereas 12.4% and 30.9% of vaccinated and unvaccinated patients, respectively, reported Black or African American race (p<0.001). Among 345,220 vaccine-eligible persons in the population who had received 1 dose of JYNNEOS vaccine, a significantly smaller percentage identified as White (46.7%) compared with vaccinated mpox patients (62.8%; p = 0.02). Overall, 259 (98.1%) vaccinated and 5,710 (96.1%) unvaccinated mpox cases occurred in persons identifying as male.
Information on at least one clinical finding was available for 202 (73.2%) of 276 vaccinated persons with mpox and 5,326 (84.2%) of 6,329 unvaccinated mpox patients. Among those who were vaccinated, the most commonly reported signs and symptoms were rash (96.5%), pruritis (33.5%), and enlarged lymph nodes (31.6%) (Table 2). Among unvaccinated persons, the most common signs and symptoms were rash (97.3%), fever (46.5%), and malaise (43.0%). The odds of fever, headache, malaise, abdominal pain, vomiting or nausea, myalgia, and chills were significantly lower among vaccinated than among unvaccinated patients (Figure). Odds of rectal signs and symptoms (e.g., proctitis, rectal bleeding, tenesmus, and rectal pain) were similar among vaccinated and unvaccinated mpox patients.
Among both vaccinated and unvaccinated patients, the genital area was the rash location most commonly reported (55.0% of vaccinated patients and 46.7% of unvaccinated patients) (Table 2). The odds of reporting rash in all other locations except the perianal area were significantly lower among vaccinated than among unvaccinated patients. The median number of rash locations reported by vaccinated patients (two) was significantly lower than that reported by unvaccinated patients (three) (p<0.001). Among 129 persons with mpox who received 1 vaccine dose ≥22 days before illness onset, demographic and clinical findings were not different from those in persons who had received vaccine ≥14 days earlier.
Among 3,142 unvaccinated persons with mpox, 237 (7.5%) were hospitalized compared with two (2.1%) of 95 vaccinated mpox patients (odds ratio = 0.27; 95% CI = 0.06–1.09). No deaths were reported in either group.
Discussion
In this analysis of 276 mpox cases in persons who received 1 dose of JYNNEOS vaccine ≥14 days before illness onset and 6,329 cases in unvaccinated persons during the 2022 U.S. outbreak, vaccinated patients reported signs and symptoms similar to those described earlier in the outbreak (1); however, some symptoms were reported less frequently among vaccinated than among unvaccinated mpox patients. In addition, the percentage of vaccinated patients who were hospitalized (2%) was lower than that among unvaccinated patients (8%), and the odds of systemic signs and symptoms, such as fever and chills, were lower among vaccinated patients. These findings indicate that 1 dose of the JYNNEOS vaccine might attenuate the severity of mpox illness in persons who are infected after vaccination.
The frequent presentation of rash in the genital and perianal areas among both vaccinated and unvaccinated mpox patients suggests that sexual transmission in this population was a common mechanism of transmission. The fewer number of reported rash locations among vaccinated patients suggests possible prevention of spread of rash from site of inoculation among even partially vaccinated persons.
The predominance of White persons among vaccinated mpox patients compared with unvaccinated patients reflects the ongoing racial and ethnic disparities in receipt of the JYNNEOS vaccine nationwide (5,6) and could indicate differential access to or acceptance of the vaccine. Disparities in access to health care and additional treatment options could also have played a role in decreasing the severity of illness in vaccinated White persons.
The findings in this report are subject to at least four limitations. First, 47% of cases were excluded because of missing vaccination information; therefore, results might not be generalizable to all persons with mpox in the United States. Second, persons with mpox who received vaccine outside of their respective jurisdictions of residence might not have had documentation of vaccine receipt, which could lead to potential undercounting of cases among vaccinated persons (7), although many jurisdictions did report sharing vaccination data with one another (start highlightSarah Gillaniend highlight, District of Columbia Department of Health, personal communication, September 2022). Third, self-reported doses were included to optimize ascertainment of vaccination status, but self-report is less accurate than documented vaccine receipt and could result in misclassification of vaccination status. Finally, clinical data were not available for all cases. In particular, HIV status was unknown or missing for two thirds of vaccinated and more than one half of unvaccinated patients. Because HIV infection might affect the trajectory and illness severity, future studies with additional data for real-world use of JYNNEOS vaccine against clinical outcomes, including data on HIV history, are needed.
The more limited distribution of rash and reduced severity of illness among persons who had mpox after receiving 1 JYNNEOS vaccine dose supports the potential benefit of vaccination on attenuation of disease. Although infection ≥14 days after receipt of 1 JYNNEOS vaccine dose is infrequent, the occurrence of such cases and the unknown duration of protection conferred by 1 vaccine dose highlights the need for providers and public health officials to encourage completion of the 2-dose vaccination series among persons at risk and continue to provide guidance and education regarding nonvaccine-related prevention strategies (4) until optimal immune protection from the second dose is achieved.
Acknowledgments
Kaitlin Grosgebauer, Linda Lewis, Timothy Lo, David Melton, Kayla Saadeh, Dhawani Shah, California Department of Public Health; Thelonious W. Williams, CDC Foundation; Alexandra Davis, Hallie Hutchison, Janna Kerins, Sarah Love, Peter Ruestow, Chicago Department of Public Health; Meghan Barnes, Emily Spence Davizon, Meenalochani Ganesan, Meghan Shea, Robyn Weber, Colorado Department of Public Health and Environment; Naga Chaitanya Alampally, Nancy L. Barrett, Thomas Bavone, Amanda J. Durante, Connecticut Department of Public Health; Andrew Hanks, Ashwin Mohana Sundaram, Chesapeake Regional Information System for Our Patients (CRISP); Staci Blum, Delaware Health and Social Services; Sarah Gillani, Michelle Lee, Karla Miletti, Allison Morrow, Michael Staff, Christina Willut, District of Columbia Department of Health; Jeremy Adams, Thomas Bendle, Danielle Stanek, Thomas Troelstrup, Florida Department of Health; Amanda Feldpausch, Jessica Pavlick, Georgia Department of Public Health; Kalie Ganem, Chanee Massiah, Lori Saathoff-Huber, Marguerite Smith, Illinois Department of Public Health; Boudu Bingay, Catherine M. Brown, Brandi Hopkins, Dylan Leach, Lawrence C. Madoff, Matthew Osborne, Elizabeth Russo, Sarah Scotland, Massachusetts Department of Public Health; Teri Adams, Jim Collins, Joe Coyle, Pauline Harrington, Shannon Johnson, Abhinav Nalla, Michigan Department of Health and Human Services; Jayne Griffith, Rachel Ostadkar, Jeff Sanders, Yeng Vang, Minnesota Department of Health; Monica Beddo, Missouri Department of Health and Senior Services; Margaret Cook-Shimanek, Trisha Gardner, Beth Hopkins, Jessica Lopeman, Montana Department of Public Health and Human Services; Samir Koirala, Kyle Strand, Nebraska Department of Health and Human Services; Kathryn Cruz, Mika Gehre, New Mexico Department of Health; Bridget J Anderson, Bryon Backenson, Youjung Byun, Eli Rosenberg, Jamie Sommer, New York State Department of Health; Amanda Timmons, Oregon Health Authority, Public Health Division; Beth Butler, Jill Garland, Tom McCleaf, Lisa McHugh, Atmaram Nambiar, Mia Russo, Kirsten Waller, Pennsylvania Department of Health; Division of Disease Control Monkeypox Response Staff Members, City of Philadelphia Department of Public Health; Miledis Sanchez Archilla, Elvis Nieves Miranda, Puerto Rico Department of Health; Utpala Bandy, Abby Berns, Suzanne Bornschein, Kevin Cormier, Dhwani Dave, Stephanie Doyle, Clement Forbes, Christine Goulette, Lara Grenier, Brandi Hansen, Karen Luther, Patricia McAuley, Elizabeth Nahod, Nancy Persson, Amanda Prymak, Daniela Quilliam, Bill Rebuck, Alyson Thurber, Nairobi Vina, Rhode Island Department of Health; Rachel Burri, Karen Butts, Kenzie Chase, Adrienne Davis, Susan Durham, Victoria Greer, Ashlyn Lancaster, Ellen Mays, LaShonda McElveen, Tammy McFarland, MyLinda O’Quinn, Caitlin Revell, Elizabeth Reynolds, Jennifer Sanders, Elizabeth Sclater, Tonya Shipman, Rebecca Whisenhunt, Tracey Yazvac, South Carolina Department of Health and Environmental Control; Greg Chambers, Rachel Wofford, Tennessee Department of Health; Jason Barnes, Jake Tant-Arrillaga, Utah Department of Health and Human Services; Brandy Darby, Ozzie DeLoach, Lori Flammia, Aaron Silverstein, Phillip Terrono, Caroline Wood, Virginia Department of Health; Yuri Bonilla, Alex Cox, Whitney Harrison, Mariana Rosenthal, Chelsea Stacy, Melinda Tran, Chunyi Wu, Washington State Department of Health; Suzanne Gibbons-Burgener, Christopher Steward, Wisconsin Department of Health Services; Wisconsin local and tribal health agencies.
Mpox Cases in Vaccinated Persons Team
Matthew Cole; Lauren Roper; Hazel Shah, CDC 2022 Multinational Monkeypox Vaccine Effectiveness Team CDC 2022 Multinational Monkeypox Informatics Team CDC 2022 Multinational Monkeypox Vaccine Data Team; Louise McNitt, California Department of Public Health; Stephanie Gretsch, Chicago Department of Public Health; Melissa Pike, Colorado Department of Public Health and Environment; Patricia Firmender, Connecticut Department of Public Health; Will Still, District of Columbia Department of Health; Jamie Ahlers, Delaware Health and Social Services; Aman Punwani, Florida Department of Health; Komal Patel, Georgia Department of Public Health; Nam-Kyu Cho, Illinois Department of Public Health; Marcia Pearlowitz, Maryland Department of Health; Petra Schubert, Massachusetts Department of Public Health; Ryan Malosh, Michigan Department of Health and Human Services; Sydney Kuramoto, Minnesota Department of Health; Matthew Donahue, Nebraska Department of Health and Human Services; Miranda Durham, New Mexico Department of Health; Charlotte DelBarba, New York State Department of Health; Kelly Cogswell, Oregon Health Authority, Public Health Division; Julie Miedlar, Pennsylvania Department of Health; Dana Perella, City of Philadelphia Department of Public Health; Julian D. Cordero Calderon, Puerto Rico Department of Health Monkeypox Team at the Center for Acute Infectious Disease Epidemiology at Rhode Island Department of Health; Taidy Perez, South Carolina Department of Health and Environmental Control; Jacqueline Logan, Tennessee Department of Health; Abigail Collingwood, Utah Department of Health and Human Services; Naihlah Smith, Washington State Department of Health; Rachel Klos, Wisconsin Department of Health Services.
Corresponding author: Jennifer L. Farrar, ihi4@cdc.gov.
1CDC Mpox Emergency Response Team; 2Epidemic Intelligence Service, CDC; 3California Department of Public Health; 4Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; 5Chicago Department of Public Health, Chicago, Illinois; 6Colorado Department of Public Health and Environment; 7Connecticut Department of Public Health; 8Council of State and Territorial Epidemiologists, Atlanta, Georgia; 9District of Columbia Department of Health; 10Delaware Department of Health and Social Services; 11Florida Department of Health; 12Georgia Department of Public Health; 13Illinois Department of Public Health; 14Massachusetts Department of Public Health; 15Maryland Department of Health; 16Michigan Department of Health and Human Services; 17Minnesota Department of Health; 18Missouri Department of Health and Senior Services; 19Nebraska Department of Health and Human Services; 20New Mexico Department of Health; 21New York State Department of Health; 22Oregon Health Authority, Public Health Division; 23Pennsylvania Department of Health; 24City of Philadelphia Department of Public Health, Philadelphia, Pennsylvania; 25Puerto Rico Department of Health; 26Rhode Island Department of Health; 27South Carolina Department of Health and Environmental Control; 28Tennessee Department of Health; 29Utah Department of Health and Human Services; 30Virginia Department of Health; 31Washington State Department of Health; 32Wisconsin Department of Health Services.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Emma Creegan reports receiving an honorarium for a fall 2021 speaking event at Brown University. No other potential conflicts of interest were disclosed.
* https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
† https://www.fda.gov/media/131078/download
§ California; Chicago, Illinois; Colorado; Connecticut; Delaware; District of Columbia; Florida; Georgia; Illinois; Massachusetts; Maryland; Michigan; Minnesota; Missouri; Montana; Nebraska; New Mexico; New York (not including New York City); Oregon; Pennsylvania; Philadelphia, Pennsylvania; Puerto Rico; Rhode Island; South Carolina; Tennessee; Utah; Virginia; Washington; and Wisconsin.
¶ https://www.cdc.gov/poxvirus/monkeypox/clinicians/case-definition.html
** For this analysis, persons were considered vaccinated ≥14 days after receipt of 1 dose of JYNNEOS vaccine, based on previous immunogenicity studies showing an immunologic response 14 days after vaccination.
†† https://www.cdc.gov/poxvirus/monkeypox/pdf/scrf-short-form.pdf
§§ Jurisdictions were considered to have collected symptom variables in a “check-all-that-apply” manner if a case patient had a “yes” response for one or more clinical symptoms, but all other symptoms were reported as missing.
¶¶ 5 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
*** A total of 6,856 (47.3%) cases were excluded because information on vaccination status or date of vaccination was missing, 106 (0.7%) had a vaccine date before May 2022 and were excluded, and 927 (6.4%) were excluded because they had been vaccinated <14 days from illness onset.
References
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1449–56. https://doi.org/10.15585/mmwr.mm7145a4 PMID:36355615
- Food and Drug Administration. Fact sheet for healthcare providers administering vaccine: emergency use authorization of JYNNEOS (smallpox and monkeypox vaccine, live, non-replicating) for prevention of monkeypox disease in individuals determined to be at high risk for monkeypox infection. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2022. https://www.fda.gov/media/160774/download#:~:text=The%20FDA%20has%20granted%20an,high%20risk%20for%20monkeypox%20infection
- Payne AB, Ray LC, Kugeler KJ, et al. Incidence of monkeypox among unvaccinated persons compared with persons receiving ≥1 JYNNEOS vaccine dose—32 U.S. jurisdictions, July 31–September 3, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1278–82. https://doi.org/10.15585/mmwr.mm7140e3 PMID:36201401
- CDC. Mpox vaccination basics. Atlanta, GA: US Department of Health and Human Services; CDC; 2022. Accessed December 7, 2022. https://www.cdc.gov/poxvirus/monkeypox/vaccines/vaccine-basics.html
- CDC. Technical report 3: multi-national monkeypox outbreak, United States, 2022. Atlanta, GA: US Department of Health and Human Services; CDC; 2022. Accessed October 26, 2022. https://www.cdc.gov/poxvirus/monkeypox/cases-data/technical-report/report-3.html
- Kriss JL, Boersma PM, Martin E, et al. Receipt of first and second doses of JYNNEOS vaccine for prevention of monkeypox—United States, May 22–October 10, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1374–8. https://doi.org/10.15585/mmwr.mm7143e2 PMID:36301741
- Goodwille K. Seattle residents drive to Canada for monkeypox vaccine doses. Seattle, WA: KING-TV News; 2022. https://www.king5.com/article/news/health/monkeypox/seattle-residents-drive-canada-monkeypox-vaccine-doses/281-6c2fcaa4-94fb-4a5e-ab36-0ee9c9683c05
Abbreviations: mpox = monkeypox; NA = not applicable.
* California; Chicago, Illinois; Colorado; Connecticut; Delaware; District of Columbia; Florida; Georgia; Illinois; Massachusetts; Maryland; Michigan; Minnesota; Missouri; Montana; Nebraska; New Mexico; New York (not including New York City); Oregon; Pennsylvania; Philadelphia, Pennsylvania; Puerto Rico; Rhode Island; South Carolina; Tennessee; Utah; Virginia; Washington; and Wisconsin.
† Percentages were calculated using cases with available data as the denominator.
§ Cases of probable and confirmed mpox in persons with illness onset ≥14 days after receipt of 1 vaccine dose.
¶ Cases of probable and confirmed mpox in persons with illness onset before the date of vaccination or who did not report receipt of vaccine.
** Medians were compared using a Wilcoxon rank sum test. Proportions were compared using Pearson’s chi-square test or Fisher’s exact test.
†† Includes all persons within the vaccine-eligible population who received 1 vaccine dose, including vaccinated mpox patients.
§§ Comparison between recipients of 1 vaccine dose in the vaccine-eligible population and vaccinated mpox patients.
Abbreviation: mpox = monkeypox.
* Persons with complete data for one or more clinical symptom were included in analysis; for vaccinated persons 73.2% were included (202/276), and for unvaccinated persons 84.2% were included (5,326/6,329).
† California; Chicago, Illinois; Colorado; Connecticut; Delaware; District of Columbia; Florida; Georgia; Illinois; Massachusetts; Maryland; Michigan; Minnesota; Missouri; Montana; Nebraska; New Mexico; New York (not including New York City); Oregon; Pennsylvania; Philadelphia, Pennsylvania; Puerto Rico; Rhode Island; South Carolina; Tennessee; Utah; Virginia; Washington; and Wisconsin.
§ Cases of probable and confirmed mpox in persons with illness onset ≥14 days after receipt of 1 vaccine dose (202/276).
¶ Cases of probable and confirmed mpox in persons with illness onset before the date of vaccination or who did not report receipt of vaccine (5,326/6,329).
** Median rash locations reported from 160 vaccinated and 4,140 unvaccinated mpox patients.
†† Medians compared using Wilcoxon rank sum test.
 FIGURE. Odds* of signs and symptoms present among persons with mpox who received 1 dose of JYNNEOS vaccine compared with those in unvaccinated persons with mpox — 29 U.S. jurisdictions,† May 22–September 3, 2022
FIGURE. Odds* of signs and symptoms present among persons with mpox who received 1 dose of JYNNEOS vaccine compared with those in unvaccinated persons with mpox — 29 U.S. jurisdictions,† May 22–September 3, 2022
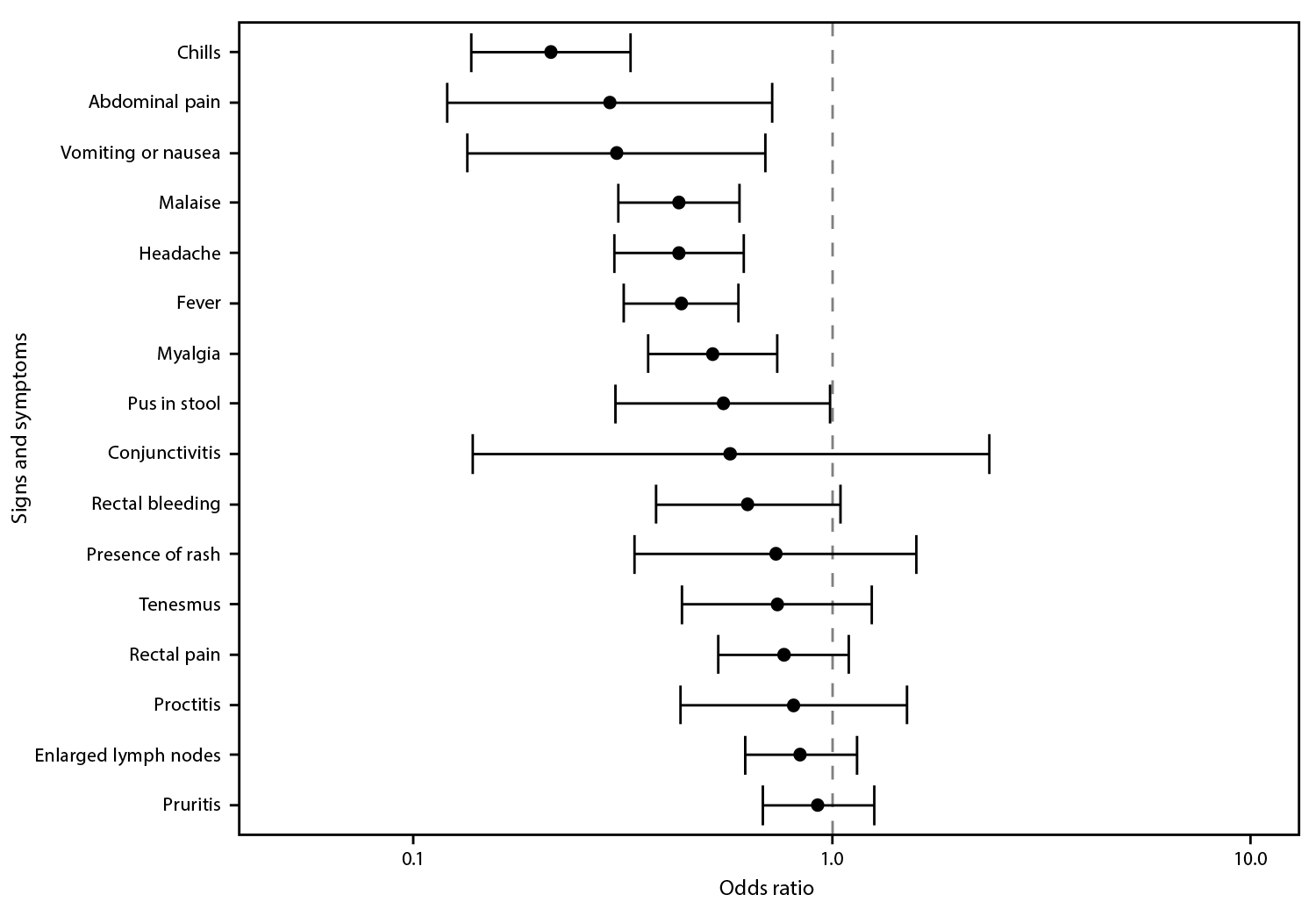
* With 95% CIs indicated by error bars.
† California; Chicago, Illinois; Colorado; Connecticut; Delaware; District of Columbia; Florida; Georgia; Illinois; Massachusetts; Maryland; Michigan; Minnesota; Missouri; Montana; Nebraska; New Mexico; New York (not including New York City); Oregon; Pennsylvania; Philadelphia, Pennsylvania; Puerto Rico; Rhode Island; South Carolina; Tennessee; Utah; Virginia; Washington; and Wisconsin.
Suggested citation for this article: Farrar JL, Lewis NM, Houck K, et al. Demographic and Clinical Characteristics of Mpox in Persons Who Had Previously Received 1 Dose of JYNNEOS Vaccine and in Unvaccinated Persons — 29 U.S. Jurisdictions, May 22–September 3, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1610–1615. DOI: http://dx.doi.org/10.15585/mmwr.mm715152a2.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
