Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season
In 2010, the Advisory Committee on Immunization Practices (ACIP) first recommended annual influenza vaccination for all persons aged ≥6 months in the United States (1). Annual influenza vaccination of all persons aged ≥6 months continues to be recommended. This document 1) describes influenza vaccine virus strains included in the U.S. seasonal influenza vaccine for 2012–13; 2) provides guidance for the use of influenza vaccines during the 2012–13 season, including an updated vaccination schedule for children aged 6 months through 8 years and a description of available vaccine products and indications; 3) discusses febrile seizures associated with administration of influenza and 13-valent pneumococcal conjugate (PCV-13) vaccines; 4) provides vaccination recommendations for persons with a history of egg allergy; and 5) discusses the development of quadrivalent influenza vaccines for use in future influenza seasons. Information regarding issues related to influenza vaccination that are not addressed in this update is available in CDC's Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010 and associated updates (1,2).
Methodology for the formulation of the ACIP annual vaccine recommendations has been described previously (1). The ACIP Influenza Work Group meets every 2–4 weeks throughout the year. Work Group membership includes several voting members of ACIP and representatives of ACIP Liaison Organizations. Meetings are held by teleconference and include discussion of influenza-related issues, such as influenza surveillance, vaccine effectiveness and safety, coverage in groups recommended for vaccination, program feasibility, cost-effectiveness, and anticipated vaccine supply. Presentations are requested from invited experts, and published and unpublished data are discussed. CDC's Influenza Division provides data on influenza surveillance, antiviral resistance, and vaccine effectiveness. CDC's Immunization Safety Office provides information on vaccine safety, and CDC's Immunization Services Division provides information on vaccine distribution and coverage.
Vaccine Strains for the 2012–13 Influenza Season
U.S. influenza vaccines for 2012–13 will contain A/California/7/2009 (H1N1)-like, A/Victoria/361/2011 (H3N2)-like, and B/Wisconsin/1/2010-like (Yamagata lineage) antigens. The influenza A(H3N2) and B antigens differ from the respective 2010–11 and 2011–12 seasonal vaccine antigens (3). The influenza A(H1N1) vaccine virus strain is derived from an influenza A(H1N1)pdm09 (2009[H1N1]) virus and was included in the 2009(H1N1) monovalent pandemic vaccine as well as the 2010–11 and 2011–12 seasonal vaccines.
Recommendations for Vaccination
Routine annual influenza vaccination is recommended for all persons aged ≥6 months. To permit time for production of protective antibody levels (4,5), vaccination optimally should occur before onset of influenza activity in the community. Therefore, vaccination providers should offer vaccination as soon as vaccine is available. Vaccination should be offered throughout the influenza season (i.e., as long as influenza viruses are circulating in the community).
Vaccine Dose Considerations for Children Aged 6 Months Through 8 Years
Children aged 6 months through 8 years require 2 doses of influenza vaccine (administered a minimum of 4 weeks apart) during their first season of vaccination to optimize immune response. In a study of children aged 5 through 8 years receiving trivalent inactivated influenza vaccine (TIV) for the first time, the proportion of children with protective antibody responses was significantly higher after 2 doses compared with a single dose (6). Several studies have indicated that the time interval between two initial doses (from 4 weeks up to 1 year) of the same antigen might not be critical (7–9). However, because of the antigenic novelty of the 2009(H1N1) pandemic virus, which is anticipated to continue circulating during 2012–13, exposure history to this antigen also must be considered. Children who last received seasonal (trivalent) influenza vaccine before the 2010–11 season but did not receive a vaccine containing 2009(H1N1) antigen (either seasonal vaccine since July 2010 or monovalent 2009[H1N1] vaccine) will not have received this antigen. These children are recommended to receive 2 doses this season, even if 2 doses of seasonal influenza vaccine were received before the 2010–11 season. This is illustrated in two approaches for determining the number of doses required for children aged 6 months through 8 years, both of which are acceptable (Figure 1).
1. The first approach takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. This recommendation is harmonized with that of the American Academy of Pediatrics (10). This approach has the advantage of simplicity, particularly in settings in which ascertaining vaccination history before the 2010–11 season is difficult. Using this approach, children aged 6 months through 8 years need only 1 dose of vaccine in 2012–13 if they received a total of 2 or more doses of seasonal vaccine since July 1, 2010. Children who did not receive a total of 2 or more doses of seasonal vaccine since July 1, 2010, require 2 doses in 2012–13.
2. In settings where adequate vaccination history from before the 2010–11 season is available, the second approach may be used. By this approach, if a child aged 6 months through 8 years is known to have received at least 2 seasonal influenza vaccines during any previous season, and at least 1 dose of a 2009(H1N1)-containing vaccine (i.e., either 2010–11 or 2011–12 seasonal vaccine or the monovalent 2009[H1N1] vaccine), then the child needs only 1 dose for 2012–13. Using this approach, children aged 6 months through 8 years need only 1 dose of vaccine in 2012–13 if they have received any of the following:
– 2 or more doses of seasonal influenza vaccine since July 1, 2010; or
– 2 or more doses of seasonal influenza vaccine before July 1, 2010, and 1 or more doses of monovalent 2009(H1N1) vaccine; or
– 1 or more doses of seasonal influenza vaccine before July 1, 2010, and 1 or more doses of seasonal influenza vaccine since July 1, 2010.
Children for whom one of these conditions is not met require 2 doses in 2012–13.
Available Vaccine Products and Indications
Multiple influenza vaccines (with the same antigenic composition) are expected to be available during the 2012–13 season (Table). Current package inserts should be consulted for updated information and description of additional components of various vaccine formulations, indications, contraindications, and precautions.
TIV preparations, with the exception of Fluzone Intradermal (Sanofi Pasteur), should be administered intramuscularly. For adults and older children, the deltoid is the preferred site. Infants and younger children should be vaccinated in the anterolateral thigh. Specific guidance regarding site and needle length for intramuscular administration can be found in ACIP's General Recommendations on Immunization (11). For intramuscular TIV preparations, children aged 6 through 35 months receive 0.25 mL per dose; persons aged ≥36 months receive 0.5 mL per dose (Table). Fluzone Intradermal is administered intradermally via a single-dose, prefilled microinjection syringe. The preferred site for administration is over the deltoid muscle.
Age indications for the various TIV products differ. All TIV preparations contain the same quantity of hemagglutinin (15 µg per vaccine virus strain per 0.5 mL dose; 45 µg total), except Fluzone Intradermal and Fluzone High-Dose (Sanofi Pasteur). Fluzone Intradermal is indicated for persons aged 18 through 64 years and contains 9 µg of hemagglutinin per vaccine virus strain (27 µg total) in a 0.1 mL dose. Fluzone High-Dose is indicated for persons aged ≥65 years and contains 60 µg of hemagglutinin per vaccine virus strain (180 µg total) in a 0.5 mL dose. Within specified age indications, ACIP expresses no preference for any given TIV formulation over another.
The intranasally administered live-attenuated influenza vaccine (LAIV), FluMist (MedImmune), is indicated for healthy, nonpregnant persons aged 2 through 49 years. No preference is indicated for LAIV versus TIV in this age group (1). Persons with a history of egg allergy should receive TIV rather than LAIV. Persons who care for severely immunosuppressed persons who require a protective environment should not receive LAIV given the theoretical risk for transmission of the live-attenuated vaccine virus.
Febrile Seizures Associated with TIV and PCV13
Febrile seizures are common in young children. At least one febrile seizure is experienced by 2%–5% of children, and nearly all children who have a febrile seizure recover quickly and are healthy afterwards (12). Before the 2010–11 influenza season, an increased risk for febrile seizures after TIV administration had not been observed in the United States (13,14). During the 2010–11 influenza season, CDC and the Food and Drug Administration (FDA) conducted enhanced monitoring for febrile seizures after influenza vaccination because of reports of an increased risk for fever and febrile seizures in young children in Australia associated with a 2010 Southern Hemisphere vaccine produced by CSL Biotherapies (up to nine febrile seizures per 1,000 doses) (15). Because of the findings in Australia, ACIP does not recommend the U.S.-licensed CSL Biotherapies' TIV, Afluria, for children aged <9 years (2,16) (Table).
Surveillance for U.S.-licensed influenza vaccines during the 2010–11 season subsequently detected safety signals for febrile seizures in young children after TIV administration (17,18). Further assessment determined that the increased risk was in children aged 6 months through 4 years on the day of vaccination to the day after (the 0–1 day risk window). The risk was higher when children received concomitant PCV13 (i.e., when the two vaccines are administered at the same health-care visit) and peaked at approximately age 16 months (18). No increased risk was observed in children aged ≥5 years after TIV or in children of any age after LAIV. The magnitude of the increased risk for febrile seizures in young children in the United States (<1 per 1,000 children vaccinated) was substantially lower than the risk observed in Australia in 2010 (15).
After evaluating the data on febrile seizures from the 2010–11 influenza season and taking into consideration benefits and risks of vaccination, no policy change was recommended for use of TIV or PCV13 for the 2011–12 season (16,19,20). Surveillance data on febrile seizures in young children after administration of influenza vaccine for the 2011–12 influenza season (same vaccine formulation as 2010–11) were consistent with those from the 2010–11 influenza season (CDC, unpublished data, 2012). No changes in the use of TIV or PCV13 are recommended for the 2012–13 influenza season. As stated previously, ACIP does not recommend the U.S.-licensed CSL Biotherapies' TIV, Afluria, for children aged <9 years (2,16) (Table).
Influenza Vaccination of Persons with a History of Egg Allergy
Severe allergic and anaphylactic reactions can occur in response to a number of influenza vaccine components, but such reactions are rare. All currently available influenza vaccines are prepared by means of inoculation of virus into chicken eggs. The use of influenza vaccines for persons with a history of egg allergy has been reviewed recently by ACIP (16). For the 2011–12 influenza season, ACIP recommended that persons with egg allergy who report only hives after egg exposure should receive TIV, with several additional safety measures, as described in this document. Recent examination of VAERS data indicated no disproportionate reporting of allergy or anaphylaxis after influenza vaccination during the 2011–12 season (21). For the 2012–13 influenza season, ACIP recommends the following:
1. Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive influenza vaccine, with the following additional safety measures (Figure 2):
a) Because studies published to date involved use of TIV, TIV rather than LAIV should be used (22);
b) Vaccine should be administered by a health-care provider who is familiar with the potential manifestations of egg allergy; and
c) Vaccine recipients should be observed for at least 30 minutes for signs of a reaction after administration of each vaccine dose (22).
Other measures, such as dividing and administering the vaccine by a two-step approach and skin testing with vaccine, are not necessary (22).
2. Persons who report having had reactions to egg involving such symptoms as angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention, particularly those that occurred immediately or within a short time (minutes to hours) after egg exposure, are more likely to have a serious systemic or anaphylactic reaction upon reexposure to egg proteins. Before receipt of vaccine, such persons should be referred to a physician with expertise in the management of allergic conditions for further risk assessment (Figure 2).
3. All vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available. ACIP recommends that all vaccination providers should be familiar with the office emergency plan (11).
4. Some persons who report allergy to egg might not be egg-allergic. Those who are able to eat lightly cooked egg (e.g., scrambled egg) without reaction are unlikely to be allergic. Egg-allergic persons might tolerate egg in baked products (e.g., bread or cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy (23). Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs and egg-containing foods, plus skin and/or blood testing for immunoglobulin E antibodies to egg proteins.
5. A previous severe allergic reaction to influenza vaccine, regardless of the component suspected to be responsible for the reaction, is a contraindication to future receipt of the vaccine.
Quadrivalent Influenza Vaccines
All currently available influenza vaccines are trivalent and contain A(H1N1), A(H3N2), and B viral antigens. There are two antigenically distinct lineages of influenza B viruses referred to as Victoria and Yamagata lineages (24). Immunization against B virus strains of one lineage provides limited cross-protection against strains in the other lineage (25). Because of this and the difficulty of predicting which B virus lineage will predominate during a given season, inclusion of a second influenza B vaccine virus strain in seasonal influenza vaccines has been proposed. A recent analysis indicates that the impact of such a quadrivalent vaccine could result in a modest reduction in influenza-associated outcomes, depending upon adequate vaccine supply, coverage, effectiveness, and incidence of influenza associated with the two B lineages (26).
In February 2012, FDA approved a new seasonal quadrivalent LAIV, FluMist Quadrivalent (MedImmune). This vaccine currently is not anticipated to be available until the 2013–14 influenza season, at which time it is expected to replace the currently available seasonal trivalent FluMist formulation (Table). Inactivated quadrivalent influenza vaccines currently are in development. These vaccines will be addressed in the ACIP influenza statement as they are approved and become available commercially.
Reported by
Lisa Grohskopf, MD, Timothy Uyeki, MD, Joseph Bresee, MD, Nancy Cox, PhD, Influenza Div, National Center for Immunization and Respiratory Diseases; Tom Shimabukuro, MD, Immunization Safety Office, National Center for Zoonotic and Emerging Infectious Diseases, CDC. Corresponding contributor: Lisa Grohskopf, lgrohskopf@cdc.gov, 404-639-2552.
Acknowledgments
Members of the Advisory Committee on Immunization Practices; member roster for July 2011–June 2012 available at http://www.cdc.gov/vaccines/recs/acip/members-archive/07-2011-06-2012.htm.
References
- CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59(No. RR-8).
- CDC. Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR 2010;59:989–92.
- Food and Drug Administration. Summary minutes: Vaccines and Related Biological Products Advisory Committee, February 28–29, 2012. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2012. Available at http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/bloodvaccinesandotherbiologics/vaccinesandrelatedbiologicalproductsadvisorycommittee/ucm296193.pdf. Accessed August 10, 2012.
- Gross PA, Russo C, Dran S, Cataruozolo P, Munk G, Lancey SC. Time to earliest peak serum antibody response to influenza vaccine in the elderly. Clin Diagn Lab Immunol 1997;4:491–2.
- Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis 1995;171:198–203.
- Neuzil KM, Jackson LA, Nelson J, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8 year-old children. J Infect Dis 2006;194:1032–9.
- Englund JA, Walter EB, Fairchok MP, Monto AS, Neuzil KM. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics 2005;115:1039–47.
- Walter EB, Neuzil KM, Zhu Y, et al. Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming? Pediatrics 2006;118:e570–8.
- Englund JA, Walter EB, Gbadebo A, et al. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics 2006;118:e579–85.
- American Academy of Pediatrics. Recommendations for the prevention and control of influenza in children, 2012–2013. Pediatrics 2012. In press.
- CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR-2).
- Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures, American Academy of Pediatrics. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics 2008;121:1281–6.
- Hambidge SJ, Glanz JM, France EK, et al. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA 2006;296:1990–7.
- Greene SK, Kulldorff M, Lewis EM, et al. Near real-time surveillance for influenza vaccine safety: proof-of-concept in the Vaccine Safety Datalink Project. Am J Epidemiol 2010;171:177–88.
- Australian Government Department of Health and Ageing, Therapeutic Goods Administration. Investigation into febrile reactions in young children following 2010 seasonal trivalent influenza vaccination. Woden, Australian Capital Territory: Australian Government Department of Health and Ageing; 2010. Available at http://www.tga.gov.au/pdf/alerts-medicine-seasonal-flu-100702.pdf. Accessed August 10, 2012.
- CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR 2011;60:1128–32.
- Leroy Z, Broder K, Menschik D, Shimabukuro T, Martin D. Febrile seizures after 2010–2011 influenza vaccine in young children, United States: a vaccine safety signal from the vaccine adverse event reporting system. Vaccine 2012;30:2020–3.
- Tse A, Tseng HF, Greene SK, et al. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine 2012;30:2024–31.
- Advisory Committee on Immunization Practices. General recommendations: febrile seizures. Influenza session. Presented at the Advisory Committee on Immunization Practices meeting, Atlanta, GA; June 2011.
- CDC. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010;59(No. RR-11).
- Advisory Committee on Immunization Practices. Update on influenza vaccine safety monitoring. Presented at the Advisory Committee on Immunization Practices meeting, Atlanta, GA; June 2012. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun12/03-influenza-shimabukuro.pdf. Accessed August 10, 2012.
- Kelso JM, Greenhawt MJ, Li JT. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol 2012;130:25–43.
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009;339:912–5.
- McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol 2004;78:12817–28.
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010;28:2149–56.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012;30:1993–8.
Recommendations for routine use of vaccines in children and adolescents are issued by CDC and are harmonized to the greatest extent possible with recommendations made by the American Academy of Pediatrics, the American Academy of Family Physicians (AAFP), and the American College of Obstetrics and Gynecology (ACOG). CDC recommendations for routine use of vaccines in adults are harmonized to the greatest extent possible with recommendations made by AAFP, ACOG, and the American College of Physicians. The Advisory Committee on Immunization Practices (ACIP) is chartered as a federal advisory committee to provide expert external advice and guidance to the Director of CDC on use of vaccines in the civilian population of the United States. ACIP members are named by the Secretary of the U.S. Department of Health and Human Services. ACIP recommendations become CDC policy once approved by the Director of CDC, on the date published by MMWR.
FIGURE 1. Influenza vaccine dosing algorithm for aged children 6 months through 8 years — Advisory Committee on Immunization Practices, United States, 2012–13 influenza season
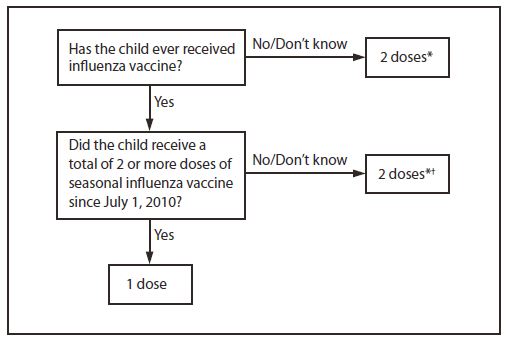
* Doses should be administered at least 4 weeks apart.
† For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. As an alternative approach in settings where vaccination history from before July 1, 2010, is available, if a child aged 6 months through 8 years is known to have received at least 2 seasonal influenza vaccines during any previous season, and at least 1 dose of a 2009(H1N1)-containing vaccine (i.e., either 2010–11 or 2011–12 seasonal vaccine or the monovalent 2009[H1N1] vaccine), then the child needs only 1 dose for 2012–13. Using this approach, children aged 6 months through 8 years need only 1 dose of vaccine in 2012–13 if they have received any of the following: 1) 2 or more doses of seasonal influenza vaccine since July 1, 2010; 2) 2 or more doses of seasonal influenza vaccine before July 1, 2010, and 1 or more doses of monovalent 2009(H1N1) vaccine; or 3) 1 or more doses of seasonal influenza vaccine before July 1, 2010, and 1 or more doses of seasonal influenza vaccine since July 1, 2010. Children for whom one of these conditions is not met require 2 doses in 2012–2013.
Alternate Text: The figure above shows influenza vaccine dosing algorithm for aged children 6 months through 8 years in the United States, during the 2012-13 influenza season. Children are recommended to receive 2 doses this season, even if 2 doses of seasonal influ¬enza vaccine were received before the 2010-11 season. This is illustrated in two approaches for determining the number of doses required for children aged 6 months through 8 years, both of which are acceptable.
|
TABLE. Influenza vaccine information, by age group — United States, 2012–13 influenza season* |
||||||||
|---|---|---|---|---|---|---|---|---|
|
Vaccine |
Trade name |
Manufacturer |
Presentation |
Mercury content (µg Hg per 0.5 mL dose) |
Ovalbumin content (µg per 0.5mL dose)† |
Age group |
No. of doses |
Route |
|
TIV |
Fluzone |
Sanofi Pasteur |
0.25 mL prefilled syringe |
0.0 |
—§ |
6–35 mos |
1 or 2¶ |
IM** |
|
0.5 mL prefilled syringe |
0.0 |
—§ |
≥36 mos |
1 or 2¶ |
IM** |
|||
|
0.5 mL vial |
0.0 |
—§ |
≥36 mos |
1 or 2¶ |
IM** |
|||
|
5.0 mL multidose vial |
25.0 |
—§ |
≥6 mos |
1 or 2¶ |
IM** |
|||
|
TIV |
Agriflu |
Novartis Vaccines |
0.5 mL prefilled syringe |
0 |
<0.4 |
≥18 yrs |
1 |
IM** |
|
TIV |
Fluvirin |
Novartis Vaccines |
0.5 mL prefilled syringe |
≤1 |
≤1 |
≥4 yrs |
1 or 2¶ |
IM** |
|
5.0 mL multidose vial |
25.0 |
≤1 |
||||||
|
TIV |
Fluarix |
GlaxoSmithKline |
0.5 mL prefilled syringe |
0 |
≤0.05 |
≥3 yrs |
1 or 2¶ |
IM** |
|
TIV |
FluLaval |
ID Biomedical Corporation of Quebec (distributed by GlaxoSmithKline) |
5.0 mL multidose vial |
<25.0 |
≤0.3 |
≥18 yrs |
1 |
IM** |
|
TIV |
Afluria |
CSL Biotherapies (distributed by Merck) |
0.5 mL prefilled syringe |
0.0 |
≤1 |
≥9 yrs†† |
1 |
IM** |
|
5.0 mL multidose vial |
24.5 |
≤1 |
||||||
|
TIV high- dose§§ |
Fluzone High-Dose |
Sanofi Pasteur |
0.5 mL prefilled syringe |
0.0 |
—§ |
≥65 yrs |
1 |
IM** |
|
TIV intradermal¶¶ |
Fluzone Intradermal |
Sanofi Pasteur |
0.1 mL prefilled microinjection system |
0.0 (per 0.1 mL) |
—§ |
18–64 yrs |
1 |
ID |
|
LAIV |
FluMist*** |
MedImmune |
0.2 mL prefilled intranasal sprayer |
0.0 (per 0.2 mL) |
<0.24 (per 0.2mL)††† |
2–49 yrs§§§ |
1 or 2¶ |
IN |
|
Abbreviations: TIV = trivalent inactivated vaccine; LAIV = live-attenuated influenza vaccine; IM = intramuscular; ID = intradermal; IN = intranasal. * Vaccination providers should consult Food and Drug Administration–approved prescribing information for 2012–13 influenza vaccines for the most updated information, including indications, contraindications, and precautions. † Data on maximum ovalbumin content is supplied in package inserts of certain vaccines. Persons with a history of mild allergy to egg (specifically, those who experience only hives) should receive TIV with additional precautions (Figure 2). § Information is not included in package insert but is available upon request from the manufacturer, Sanofi Pasteur, by contacting 1-800-822-2463 or mis.emails@sanofipasteur.com. ¶ Figure 1 describes two approaches for determining the number of doses needed for children aged 6 months through 8 years. ** For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. †† Age indication per package insert is ≥5 years; however, the Advisory Committee on Immunization Practices recommends that Afluria not be used in children aged 6 months through 8 years because of increased risk for febrile reactions noted in this age group with CSL's 2010 Southern Hemisphere TIV. If no other age-appropriate, licensed inactivated seasonal influenza vaccine is available for a child aged 5 through 8 years who has a medical condition that increases the child's risk for influenza complications, Afluria can be used; however, vaccination providers should discuss with the parents or caregivers the benefits and risks of influenza vaccination with Afluria before administering this vaccine. Afluria may be used in persons aged ≥9 years. §§ A 0.5-mL dose contains 60 µg of each vaccine antigen (180 µg total). ¶¶ A 0.1-mL dose contains 9 µg of each vaccine antigen (27 µg total). *** A new quadrivalent formulation of FluMist was approved by the Food and Drug Administration in February 2012. It is anticipated that this formulation will replace the currently available seasonal trivalent LAIV formulation for the 2013–14 season. FluMist is shipped refrigerated and stored in the refrigerator at 35°F–46°F (2°C–8°C) after arrival in the vaccination clinic. The dose is 0.2 mL divided equally between each nostril. Health-care providers should consult the medical record, when available, to identify children aged 2 through 4 years with asthma or recurrent wheezing that might indicate asthma. In addition, to identify children who might be at greater risk for asthma and possibly at increased risk for wheezing after receiving LAIV, parents or caregivers of children aged 2 through 4 years should be asked, "In the past 12 months, has a health-care provider ever told you that your child had wheezing or asthma?" Children whose parents or caregivers answer "yes" to this question and children who have asthma or who had a wheezing episode noted in the medical record within the past 12 months should not receive FluMist. ††† Insufficient data available for use of LAIV in egg-allergic persons. §§§ Flumist is indicated for healthy, nonpregnant persons aged 2 through 49 years. Persons who care for severely immunosuppressed persons who require a protective environment should not receive FluMist given the theoretical risk for transmission of the live-attenuated vaccine virus. |
||||||||
FIGURE 2. Recommendations regarding influenza vaccination for persons who report allergy to eggs — Advisory Committee on Immunization Practices, United States, 2012–13 influenza season
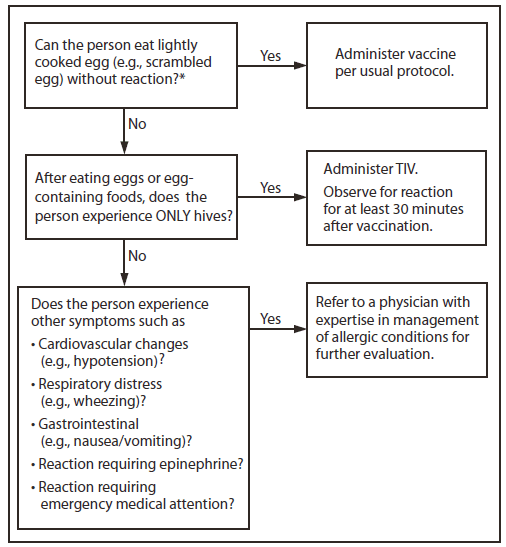
Abbreviation: TIV = trivalent inactivated vaccine.
* Persons with egg allergy might tolerate egg in baked products (e.g., bread or cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Alternate Text: The figure above shows recommendations regarding influenza vaccination for persons who report a history of egg allergy, in the United States during the 2012-13 influenza season. Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive influenza vaccine, with the use of additional safety measures.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


